Centre of Agricultural Biochemistry and Biotechnology, University of Agriculture Faisalabad, Pakistan
Centre of Agricultural Biochemistry and Biotechnology, University of Agriculture Faisalabad, Pakistan
 Author
Author  Correspondence author
Correspondence author
Bioscience Methods, 2012, Vol. 3, No. 9 doi: 10.5376/bm.2012.03.0009
Received: 21 Oct., 2012 Accepted: 29 Oct., 2012 Published: 14 Nov., 2012
Ijaz et al., 2012, Orange juice, a natural source for enhancing in vitro regeneration in Saccharum spp., Bioscience Methods, Vol.3, No.9 55-57 (doi: 10.5376/bm.2012.03.0009)
Orange juice is a natural source of various vitamins especially Vitamin C, as well as sugar, potassium, thiamine, folate, flvonoids and an antioxidant hesperidins. Therefore the effect of orange juice on in vitro regeneration of sugarcane was investigated. For this study, genotype S-2003-us-359 was selected, in which we had already established in vitro regeneration system and a media combination had been selected with highest regeneration potential. In this study, this media combination was used as control and also modified the regeneration medium of this media combination by adding orange juice and dry milk. An increase in regeneration potential of this media combination was observed by adding orange juice in regeneration medium.
Introduction
Globally, sugarcane has got a prime candidate as a cash crop which fulfilled 80% world sugar demand and emerging biofuel crop in near future due to its dexterous biomass production (Gallo-Meagher et al., 2000). This tropical grass is the member of family Poaceae which contains high polyploidy level (2n=80~270) (Heinz and Mee, 1969). This grass is the most suitable promising crop which could be utilized mainly for sugar production and then for power generation, paper making, live stock feed, chipboard, cane wax, fertilizer, bioethanol, syrup and mulch. Recently, additional revenue being produced in the form of bioplastics by sugarcane stalk and bagasse from sugarcane. Sugarcane as a perennial crop is the world’s most resourceful crop in converting solar energy into chemical energy. The preliminary but significant results led to the utilization of in vitro cell and tissue culture for a range of functions such as micropropagation (Hendre et al., 1983), breeding (Krishnamurthy and Tlaskal, 1974), germplasm conservation (Taylor and Dukic, 1993), the eradication of systemic pathogens (Parmessur et al., 2002) and genetic engineering (Arencibia et al., 1995) of sugarcane. (Taylor et al., 1992) tested sugarcane cultivars readily produce embryogenic callus from young leaf tissue and readily regenerate plants from callus. Plant tissue culture techniques have become a powerful tool for studying and solving basic and applied problems in plant biotechnology especially gene introduction (Villalobos and Arias, 1987). Therefore, it is significant for every genotype to optimize media with different levels of plant growth regulators along with growing conditions so that optimum regeneration could be attained (Anjum et al., 2012). Lack of suitable multiplication procedure and contamination by systemic diseases are the serious problem to multiply an elite genotype of sugarcane in the open field.
Problems associated with the traditional breeding methods have been overcome by the use of plant tissue culture techniques for the propagation of sugarcane and these techniques ensure disease free multiplication of the plants by reducing the time period required for the multiplication (Khan et al., 2006). Large-scale production of disease-free quality planting material of sugarcane can also be achieved through micropropagation. Use of plant tissue culture techniques reduces the breeding cycle (Lloyd and Pillay, 1980).
1 Results and Discussion
Lack of proficient and reproducible regeneration system to produce transformed plants at an adequate rate is still the key factor seriously restricting the improvement of crops through genetic transformation (Popelka and Alpeter, 2003). Tissue culture is a powerful tool for studying and solving, problems in plant biotechnology. A tissue culture system provides considerable quantities of target tissue.
To check the response of fresh orange juice and dry milk on in vitro regeneration, we used selected media combination that we were reported already (Ijaz et al., 2012). This media combination consisted of CIM3 (Callus induction medium 3), genotype; S-2003-us- 359, Callus age; 21 days old calli and RM3 (Regeneration medium 3). CIM3 contained 3 mg/L 2, 4-D, 40 g/L sucrose and basal MS salt. RM3 contained 0.1 mg/L 2, 4-D, 0.25 mg/L BAP, 40 g/L sucrose and basal MS salt. We just modified the RM3 in this media combination by adding orange juice RM3 (a) and dry milk RM3 (b). Calli of genotype S-2003-us-359 were induced on CIM3 and 21 days old calli were transferred to RM3 (control), RM3 (a) (modified regeneration medium by adding orange juice) and RM3 (b) (modified regeneration medium by adding dry milk) (Figure 1).
|
|
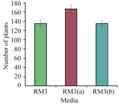
The analysis of variance (ANOVA) table depicts the significant effect of regeneration media on in vitro regeneration (Table 1). This comparative study revealed that the RM3 (a) (modified RM3 by adding orange juice) gave maximum number of plants (Figure 2) the mean value 166.33 shoots per explant. This response of RM3 (a) was statistically significantly different from control (RM3) and RM3 (b); modified RM3 by adding dry milk. But RM3 and RM3 (b) are statistically similar with mean value of 134.67 and 137.77 shoots per explant respectively (Table 2, Figure 3).
|
|
|
|
|
|
|
|
Plant juices and liquid endosperms have been shown to possess stimulatory properties. The inclusion of liquid endosperm from immature corn (Netien et al, 1951), tomato juice (Nitsch, 1951; Straus and La Rue, 1954), immature fruits and seeds (Steward and Caplin,1952; Steward and Shantz, 1959), malt extract, yeast extract, leaf extracts from a number of plants and tumor extracts (Butenko, 1968), have been reported. These results are agreed with our results because by adding orange juice in our selected regeneration RM3), the regeneration response is enhanced.
2 Materials and Methods
The immature young leaves of sugarcane genotype S-2003-us-359 were cut into slices by using blades and scalpels and were cultured on callus induction medium containing 3 mg/L 2, 4-D, 40 g/L sucrose plus MS Salt in common. They were kept on dark conditions for callus induction.
Twenty one days old calli of this genotype were shifted on regeneration media. In this experiment regeneration medium reported by us (Ijaz et al., 2012) was modified by adding orange juice and dry milk (Table 3). Here reported regeneration medium was used as control. Data were collected on the basis of number of plants. Experiment was repeated three times.
|
|
Reference
Anjum N., Ijaz S., Rana I.A., Khan T.M., Khan I.A., Khan M.N., Mustafa G., Joyia F.A., and Iqbal A., 2012, Establishment of an in vitro regeneration system as a milestone for genetic transformation of sugarcane (Saccharum officinarum L.) against Ustilago scitaminea, Bioscience Methods, 3(2): 7-20
Arencibia A., P.R. Molin G. de la Riva, and G. Selman-Housein, 1995, Production of transgenic sugarcane (Saccharum officinarum L.) plants by intact cell electroporation, Plant cell Rep., 14(5): 305-309
http://dx.doi.org/10.1007/BF00232033
Butenko R.G., 1968, In: plant tissue culture and plant morphogenesis, Isr. Prog. For Scient. Transla. Jerusalem, pp. 40-45
Gallo-Meagher M., English R.G., and Abouzid A., 2000, Thidiazuron stimulates shoot regeneration of sugarcane embryogenic callus, In vitro cell and dev. Boil. Plant, 36(1): 37-40
Hendre N.A., Iyor R.S., Kotwalm M., Kluspe S.S., and Mascarenhas A.F., 1983, Rapid multiplication of sugarcane by tissue culture, Sugarcane, pp.5-8
Ijaz S., Rana I.A., Khan I.A., and Saleem M., 2012, Establishment of an in vitro regeneration system for genetic transformation of selected sugarcane genotypes, Genet. Mol. Res., 11(1): 512-530
http://dx.doi.org/10.4238/2012.March.6.4
Khan M.S., Usman M., and Lilla M.I., 2006, Facile plant regeneration from tomato leaves induced with spectinomycin, Pak. J. Bot., 38(4): 947-952
Krishnamurthy M., and Tlaskal J., 1974, Fiji disease resistant Saccharum officinarum var. Pindar sub-clones from tissue cultures, Proc. Int. Soc. Sugar Cane Technol., 15: 130-137.
Lloyd H.L., and Pillay M., 1980, The development of an improved method for evaluating sugarcane for resistance to smut, Proceedings of the south african sugar technologists' association, 168-172
Netien G, Beauchesne G, and Mentzer C, 1951, Influence du "lait de mais" sur la croissance des tissues de carotte in vitro, Comp. Rendus de L'Acad. Des Sci., 233: 92-98
Nitsch J.P., 1951, Growth and development in vitro of excised ovaries, Am. J. Bot., 38(7): 566-571
http://dx.doi.org/10.2307/2438018
Parmessur Y., Aljanabi S., Saumtally S., and Dookun-Saumtally A., 2002, Sugarcane yellow leaf virus and sugarcane yellows phytoplasma: elimination by tissue culture, Plant Pathol., 51(5): 561-566
http://dx.doi.org/10.1046/j.1365-3059.2002.00747.x
Popelka J. C., and Altpeter F., 2003, Evaluation of rye (Secale cereale L) inbred lines and their crosses for tissue culture response and stable genetic transformation of homozygous rye inbred line L22 by biolistic gene transfer, Theor. Appl. Genet., 107(4): 583-590
http://dx.doi.org/10.1007/s00122-003-1314-0
Steward F.C., and Caplin S.M., 1952, Investigation on growth and metabolism of plant cells: Evidence on the role of coconut milk factor in development, Ann. Bot., 16(4):491-504
Steward F.C., and Shantz E.M., 1959, The chemical regulation of growth (some substances and extracts which include growth and morphogenesis), Ann. Rev. Plant. Physiol., 10: 379-404
http://10.1146/annurev.pp.10.060159.002115
Straus J., and La Rue C.D., 1954, Maize endosperm tissue growth in vitro I. Cultural requirements, Am. Bot. S., 41(8): 687-694
Taylor P.W.J, and Dukic S., 1993, Development of an in vitro culture technique for conservation of Saccharum spp. hybrid germplasm, Plant Cell Tiss. Organ Cult., 34: 217–222
http://10.1007/BF00036105
Taylor P.W.J., KO L.H., Adkins S.W., Rathus C., and Birch R.G., 1992, Establishment of embryogenic callus and high protoplast yielding suspension cultures of sugarcane (Saccharum spp. Hybrid), Plant. Cell Tiss. Org. Cult., 28(1):69-78
http://10.1007/BF00039917
Villalobos I., and Arias O., 1987, Induccion Y multipucacion de callos in vitro en tres cultiv ares comerciales de caña de azúcar (Saccharnm spp.), Agron. Costarricense, 11(1): 39-44
. PDF(305KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Siddra Ijaz
. Iqrar Ahmad Rana
. Iqrar Ahmad Khan
Related articles
. Orange juice
. Sugarcane
. In vitro regeneration
Tools
. Email to a friend
. Post a comment